One nanometer (nm) is one billionth, or 10-9 of a meter. By comparison, typical carbon-carbon bond lengths, or the spacing between these atoms in a molecule, are in the range 0.12-0.15 nm, and a DNA double-helix has a diameter around 2 nm. On the other hand, the smallest cellular lifeforms, the bacteria of the genus Mycoplasma, are around 20000 nm in length.
To put that scale in another context, the comparative size of a nanometer to a meter is the same as that of a marble to the size of the earth. Or another way of putting it: a nanometer is the amount a man's beard grows in the time it takes him to raise the razor to his face.
Two main approaches are used in nanotechnology. In the "bottom-up" approach, materials and devices are built from molecular components which assemble themselves chemically by principles of molecular recognition. In the "top-down" approach, nano-objects are constructed from larger entities without atomic-level control.
Larger to smaller: a materials perspective
A number of physical phenomena become pronounced as the size of the system decreases. These include statistical mechanical effects, as well as quantum mechanical effects, for example the “quantum size effect” where the electronic properties of solids are altered with great reductions in particle size. This effect does not come into play by going from macro to micro dimensions. However, it becomes dominant when the nanometer size range is reached. Additionally, a number of physical (mechanical, electrical, optical, etc.) properties change when compared to macroscopic systems. One example is the increase in surface area to volume ratio altering mechanical, thermal and catalytic properties of materials. Novel mechanical properties of nanosystems are of interest in the nanomechanics research. The catalytic activity of nanomaterials also opens potential risks in their interaction with biomaterials.
Materials reduced to the nanoscale can show different properties compared to what they exhibit on a macroscale, enabling unique applications. For instance, opaque substances become transparent (copper); stable materials turn combustible (aluminum); solids turn into liquids at room temperature (gold); insulators become conductors (silicon). A material such as gold, which is chemically inert at normal scales, can serve as a potent chemical catalyst at nanoscales. Much of the fascination with nanotechnology stems from these quantum and surface phenomena that matter exhibits at the nanoscale.
Simple to complex: a molecular perspective
Modern synthetic chemistry has reached the point where it is possible to prepare small molecules to almost any structure. These methods are used today to produce a wide variety of useful chemicals such as pharmaceuticals or commercial polymers. This ability raises the question of extending this kind of control to the next-larger level, seeking methods to assemble these single molecules into supramolecular assemblies consisting of many molecules arranged in a well defined manner.
These approaches utilize the concepts of molecular self-assembly and/or supramolecular chemistry to automatically arrange themselves into some useful conformation through a bottom-up approach. The concept of molecular recognition is especially important: molecules can be designed so that a specific conformation or arrangement is favored due to non-covalent intermolecular forces. The Watson-Crick basepairing rules are a direct result of this, as is the specificity of an enzyme being targeted to a single substrate, or the specific folding of the protein itself. Thus, two or more components can be designed to be complementary and mutually attractive so that they make a more complex and useful whole.
Such bottom-up approaches should be able to produce devices in parallel and much cheaper than top-down methods, but could potentially be overwhelmed as the size and complexity of the desired assembly increases. Most useful structures require complex and thermodynamically unlikely arrangements of atoms. Nevertheless, there are many examples of self-assembly based on molecular recognition in biology, most notably Watson-Crick basepairing and enzyme-substrate interactions. The challenge for nanotechnology is whether these principles can be used to engineer novel constructs in addition to natural ones.
Molecular nanotechnology: a long-term view
Molecular nanotechnology, sometimes called molecular manufacturing, is a term given to the concept of engineered nanosystems (nanoscale machines) operating on the molecular scale. It is especially associated with the concept of a molecular assembler, a machine that can produce a desired structure or device atom-by-atom using the principles of mechanosynthesis. Manufacturing in the context of productive nanosystems is not related to, and should be clearly distinguished from, the conventional technologies used to manufacture nanomaterials such as carbon nanotubes and nanoparticles.
When the term "nanotechnology" was independently coined and popularized by Eric Drexler (who at the time was unaware of an earlier usage by Norio Taniguchi) it referred to a future manufacturing technology based on molecular machine systems. The premise was that molecular-scale biological analogies of traditional machine components demonstrated molecular machines were possible: by the countless examples found in biology, it is known that sophisticated, stochastically optimised biological machines can be produced..
It is hoped that developments in nanotechnology will make possible their construction by some other means, perhaps using biomimetic principles. However, Drexler and other researchers have proposed that advanced nanotechnology, although perhaps initially implemented by biomimetic means, ultimately could be based on mechanical engineering principles, namely, a manufacturing technology based on the mechanical functionality of these components (such as gears, bearings, motors, and structural members) that would enable programmable, positional assembly to atomic specification (PNAS-1981). The physics and engineering performance of exemplar designs were analyzed in Drexler's book Nanosystems.
In general it is very difficult to assemble devices on the atomic scale, as all one has to position atoms are other atoms of comparable size and stickyness. Another view, put forth by Carlo Montemagno, is that future nanosystems will be hybrids of silicon technology and biological molecular machines. Yet another view, put forward by the late Richard Smalley, is that mechanosynthesis is impossible due to the difficulties in mechanically manipulating individual molecules.
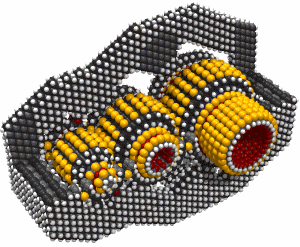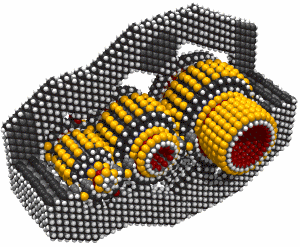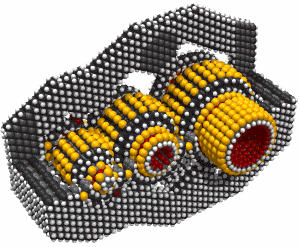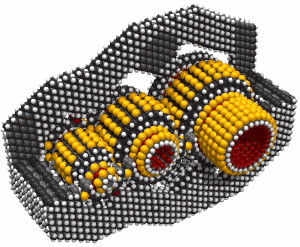
This led to an exchange of letters in the ACS publication Chemical & Engineering News in 2003. Though biology clearly demonstrates that molecular machine systems are possible, non-biological molecular machines are today only in their infancy. Leaders in research on non-biological molecular machines are Dr. Alex Zettl and his colleagues at Lawrence Berkeley Laboratories and UC Berkeley. They have constructed at least three distinct molecular devices whose motion is controlled from the desktop with changing voltage: a nanotube nanomotor, a molecular actuator, and a nanoelectromechanical relaxation oscillator.
An experiment indicating that positional molecular assembly is possible was performed by Ho and Lee at Cornell University in 1999. They used a scanning tunneling microscope to move an individual carbon monoxide molecule (CO) to an individual iron atom (Fe) sitting on a flat silver crystal, and chemically bound the CO to the Fe by applying a voltage.

No comments:
Post a Comment