While butadiyne was selected here as the primary feedstock molecule, this choice was motivated by considerations involving the simplicity and ease of analysis of the reactions and the simplicity of the binding site. Other hydrocarbon feedstocks will likely be advantageous when other criteria are used (e.g., ease of bulk production and handling of the feedstock).
Monday, September 8, 2008
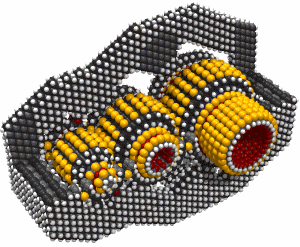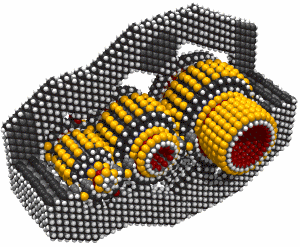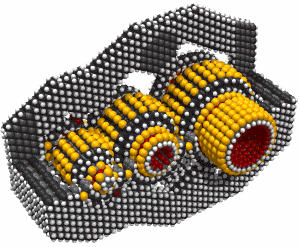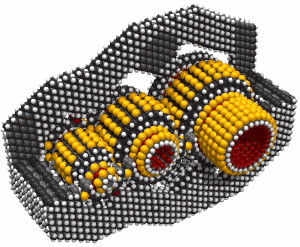
Butadiyne
Butadiyne, H-C#C-C#C-H, has a melting point of -36.4°C and a boiling point of 10.3°C. It dissolves readily in acetone. It can polymerize explosively under some conditions. The following information is from (Shostakovskii and Bogdanova, 1974), who provide rules for the safe handling of butadiyne. These include adequate dilution (less than about 30% by volume of butadiyne) and low temperatures (below about 0°C). Mixed 1:1 with butane, liquid butadiyne can be heated to a temperature of 220°C at a pressure of ~1.6 × 107 Pascals (160 atmospheres) "without any danger of explosion." At 25°C and 500 mm Hg the gas showed no evidence of polymerization after 55 days. After three months in a sealed tube at room temperature, a 7.4% solution of butadiyne in acetone showed a "floculent polymer precipitate." Various inhibitors are recommended to prevent polymerization. The heat of solution of butadiyne in acetone is 10.3 Kcal/mole in the temperature range from -40°C to +15°C. Several other solvents could also be used, including butane.
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment